Quality & InnovationExclusively Foot and Ankle
Quality & InnovationExclusively Foot and Ankle
Just LaunchedMobile Lab - Foot & Ankle Surgeon Education Brought to You
Paragon 28’s new mobile lab is housed in a 43x30 foot tractor-trailer which includes a state-of-the-art, 6-station, cadaveric training facility accommodating up to 20 surgeons. The mobile lab will host over 50 training sessions in approximately fifty US cities during the second half of 2022.
The Future of Foot & Ankle3D Planning
Disior significantly advances Paragon 28’s SMART 28 strategy, providing a comprehensive 3D analytics and pre-operative planning platform, and will immediately accelerate research.
COMPREHENSIVE SYSTEMS
Advancing the Science of Foot & Ankle Surgery
- Trauma Solutions
- HAV
- Ankle Fusion
- Biologics
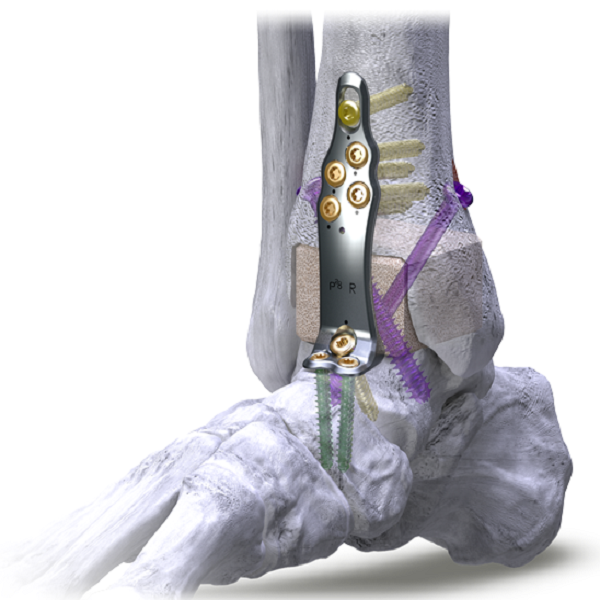
Ankle FractureGorilla® Ankle Fracture Plating System
Calcaneal FractureGorilla® Calc Fracture Plating System
Lisfranc FractureGorilla® Lisfranc Plating System
Bunion SystemPhantom® MIS System
Bunion SystemPhantom® Intramedullary Nail
Bunion SystemsLapidus Cut Guide System
Nail SystemsPhantom ActivCore™ Nail
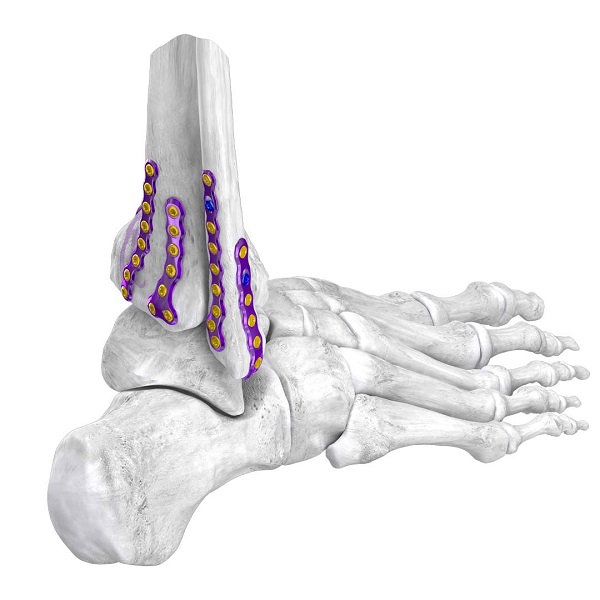
Plating SystemsSilverback™ Ankle Fusion Plating System
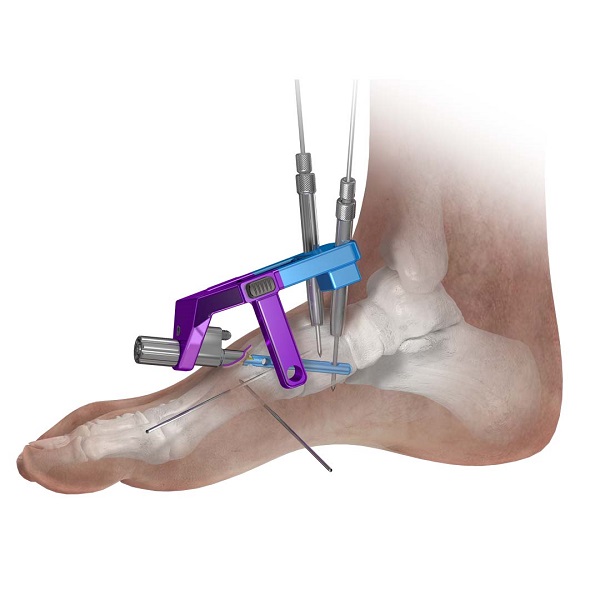
Nail SystemsPhantom® Hindfoot TTC/TC System
Bone HealingBEAST™ Demineralized Bone Matrices
Bone HealingMgnum™ BVF
Wound CarePRESERVE™ ParaDerm® Dermal Matrix
Register for an Upcoming Event
Surgeon Testimonials
Launch of Grappler® R3INFORCE™ Extraosseous Repair System
Launch of the Grappler® R3INFORCE™ Extraosseous Repair System designed to restore stability to the anterior and posterior ligaments surrounding the ankle during fibula fracture repairs and high ankle sprains.
Launch of PRECISION® MIS Bunion System
Paragon 28 announces the launch of the PRECISION® MIS Bunion System, which allows surgeons to complete a distal metatarsal osteotomy using a minimally invasive (MIS) surgical technique.
Launch of the FJ2000™ Power Console and Burr System
Paragon 28 is proud to announce the launch of its FJ2000™ Power Console and Burr System designed for a wide range of minimally invasive and open procedures.